- My Forums
- Tiger Rant
- LSU Recruiting
- SEC Rant
- Saints Talk
- Pelicans Talk
- More Sports Board
- Coaching Changes
- Fantasy Sports
- Golf Board
- Soccer Board
- O-T Lounge
- Tech Board
- Home/Garden Board
- Outdoor Board
- Health/Fitness Board
- Movie/TV Board
- Book Board
- Music Board
- Political Talk
- Money Talk
- Fark Board
- Gaming Board
- Travel Board
- Food/Drink Board
- Ticket Exchange
- TD Help Board
Customize My Forums- View All Forums
- Show Left Links
- Topic Sort Options
- Trending Topics
- Recent Topics
- Active Topics
Started By
Message
re: Increased CO2 levels produced no negative effects on GW-signed by 31,847 scientists
Posted on 3/20/19 at 10:18 am to La Place Mike
Posted on 3/20/19 at 10:18 am to La Place Mike
quote:Ah yes. Science deniers.
science deniers
We had "New Math".
Now we have "New Science" . . . . where the questioning of a hypothesis is redesignated as "denial".
Posted on 3/20/19 at 10:20 am to MastrShake
Yes, the water cycle is 3rd grade stuff. But YOU are the one who obviously didn't understand it till you looked it up due to your stupid assertion about global heat increases causing drought increases.
During the Little Ice Age (preceded and followed by warmer periods) the amount of water stayed the same.
During the Little Ice Age (preceded and followed by warmer periods) the amount of water stayed the same.
Posted on 3/20/19 at 10:26 am to djmicrobe
quote:
Signatories are approved for inclusion in the Petition Project list if they have obtained formal educational degrees at the level of Bachelor of Science or higher in appropriate scientific fields. The petition has been circulated only in the United States.
The current list of petition signers includes 9,029 PhD; 7,157 MS; 2,586 MD and DVM; and 12,715 BS or equivalent academic degrees. Most of the MD and DVM signers also have underlying degrees in basic science.
Posted on 3/20/19 at 10:44 am to NC_Tigah
This is a chart I created from raw NOAA data:
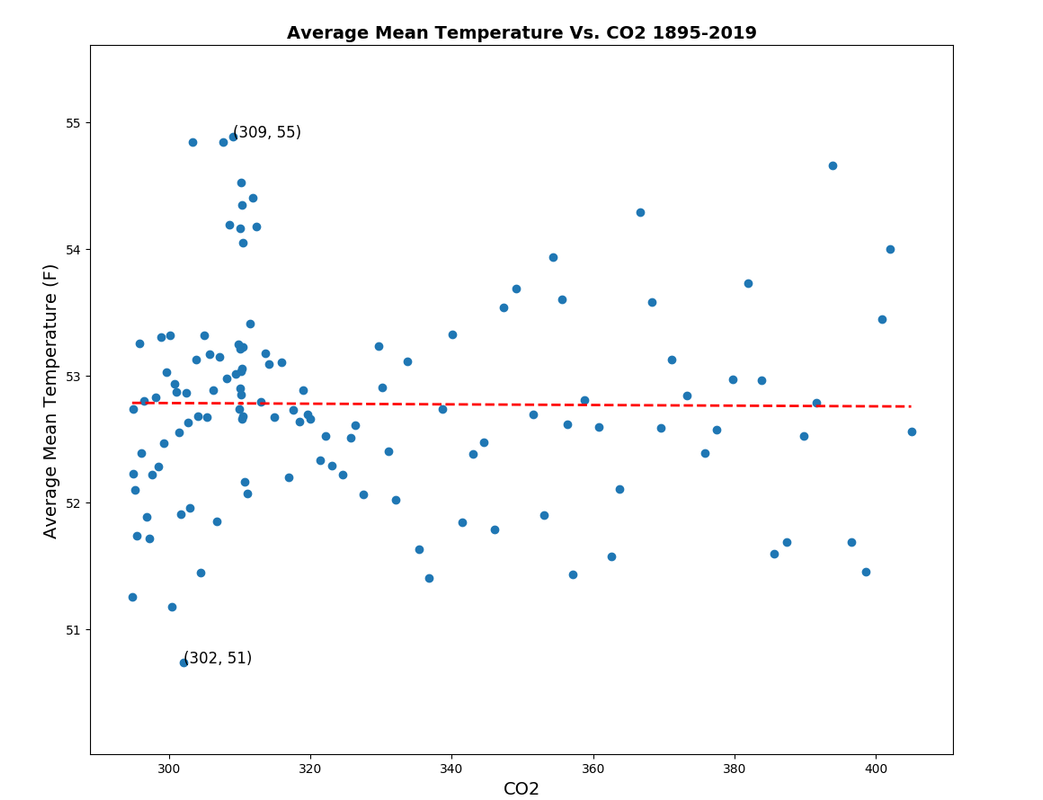
Clearly as the concentration of CO2 increases in the atmospheric mixture the temperature decreases. This can be predicted and modeled by Dalton's Law but instead of using pressure, use specific heat of each gas in the atmospheric mixture.
So what are the specific heats of the main constituents of air: nitrogen @ 78%, oxygen @ 20%, CO2 @ 0.35%, water vapor varies from 0.01 to 5% and the remaining trace gasses. I'll ignore the trace gases to keep things simple.
So the heat capacities of each gas at atmospheric pressure from highest to lowest are as follows:
Water vapor: 1.93 (kJ/(kg K))
Nitrogen: 1.04 (kJ/(kg K))
Oxygen: 0.919 (kJ/(kg K))
Carbon Dioxide: 0.844 (kJ/(kg K))
Water vapor clearly has the most ability to trap energy (i.e., heat) and CO2 has the least ability to trap energy of all the gases in the atmosphere.
Since carbon dioxide has the lowest heat capacity of the primary gas mixture in our atmosphere you would expect by increasing carbon dioxide concentration in our atmosphere that the average mean temperature would decrease. That's what NOAA's data shows.


Clearly as the concentration of CO2 increases in the atmospheric mixture the temperature decreases. This can be predicted and modeled by Dalton's Law but instead of using pressure, use specific heat of each gas in the atmospheric mixture.
So what are the specific heats of the main constituents of air: nitrogen @ 78%, oxygen @ 20%, CO2 @ 0.35%, water vapor varies from 0.01 to 5% and the remaining trace gasses. I'll ignore the trace gases to keep things simple.
So the heat capacities of each gas at atmospheric pressure from highest to lowest are as follows:
Water vapor: 1.93 (kJ/(kg K))
Nitrogen: 1.04 (kJ/(kg K))
Oxygen: 0.919 (kJ/(kg K))
Carbon Dioxide: 0.844 (kJ/(kg K))
Water vapor clearly has the most ability to trap energy (i.e., heat) and CO2 has the least ability to trap energy of all the gases in the atmosphere.
Since carbon dioxide has the lowest heat capacity of the primary gas mixture in our atmosphere you would expect by increasing carbon dioxide concentration in our atmosphere that the average mean temperature would decrease. That's what NOAA's data shows.
Posted on 3/20/19 at 11:26 am to GumboPot
quote:
Clearly as the concentration of CO2 increases in the atmospheric mixture the temperature decreases.
Clearly your plot has no correlation.
Using just the raw data isn't going to show you an accurate portrayal either, since the methods (time of day, buckets from the ocean vs ship intake) have changed over time too. I'm sure you'll have issue with the adjustments and record, but that's a different discussion.
quote:
This can be predicted and modeled by Dalton's Law but instead of using pressure, use specific heat of each gas in the atmospheric mixture.
We've done this song and dance before, but why not go again?
The heat capacity of the atmosphere is not the concern of the "greenhouse" effect. It's about how much more IR radiation is being reflected back to the surface vs out to space. Not all molecules are the same in terms of being able to absorb and then re-emit the energy.
You've seen a water vapor satellite image I presume? It works because water vapor absorbs a specific range of wavelengths. The satellite receives less of that wavelength coming to it, there's more water vapor in the upper levels. The same is done for CO2 concentration data from satellite.
There's no oxygen or nitrogen equivalent, because it's invisible to IR radiation. It passes straight on through.
The heat capacity doesn't relate to the greenhouse effect. It is about how certain molecules absorb and re-emit IR radiation being emitted by the surface.
Posted on 3/20/19 at 11:47 am to Duke
quote:
The heat capacity doesn't relate to the greenhouse effect. It is about how certain molecules absorb and re-emit IR radiation being emitted by the surface.
Regardless of wavelength (IR is not the only radiation), how much energy can each molecule absorb?
Posted on 3/20/19 at 12:21 pm to GumboPot
IR is the spectrum in question because it's what the surface emits toward space.
It's not that the greenhouse gases just absorb the heat though. They absorb it, vibrate, and re-emit it. In all directions, some of which goes back to the surface. The surface thus radiates less heat back to space overall, and warms. The surface being the main heat source for the lower atmosphere.
It's not that the greenhouse gases just absorb the heat though. They absorb it, vibrate, and re-emit it. In all directions, some of which goes back to the surface. The surface thus radiates less heat back to space overall, and warms. The surface being the main heat source for the lower atmosphere.
Posted on 3/20/19 at 12:32 pm to Duke
quote:
IR is the spectrum in question because it's what the surface emits toward space.
It's not that the greenhouse gases just absorb the heat though. They absorb it, vibrate, and re-emit it. In all directions, some of which goes back to the surface. The surface thus radiates less heat back to space overall, and warms. The surface being the main heat source for the lower atmosphere.
So you are saying that nitrogen and oxygen store no energy in our atmosphere?
Posted on 3/20/19 at 12:44 pm to GumboPot
quote:
So you are saying that nitrogen and oxygen store no energy in our atmosphere?
Of course not. I'm saying they don't absorb the radiation coming in from the sun or coming from the surface. O2 and N2 are still moving around, changing temperatures with pressure changes, ect.
Posted on 3/20/19 at 12:50 pm to Duke
quote:
Of course not. I'm saying they don't absorb the radiation coming in from the sun or coming from the surface. O2 and N2 are still moving around, changing temperatures with pressure changes, ect.
So O2 and N2 can only store energy and change temperature is via pressure changes (PV=nRT)?
Just asking questions here so please don't take this as me being a dick or trying for a gotcha.
Posted on 3/20/19 at 1:10 pm to Duke
quote:Right. We have a wonderful handle on that science in the broad environmental interface. We know for example, H2O in the atmosphere is a very import radiative forcing agent or GHA. In fact it's postulated that water vapor accounts for up to 75% of the greenhouse effect.
It's not that the greenhouse gases just absorb the heat though. They absorb it, vibrate, and re-emit it.
. . . And of course that is why deserts are so hot.
Posted on 3/20/19 at 1:11 pm to GumboPot
quote:
Just asking questions here so please don't take this as me being a dick or trying for a gotcha.
I haven't known you to argue in bad faith.
quote:
So O2 and N2 can only store energy and change temperature is via pressure changes (PV=nRT)?
There's convection from the surface to consider, mixing the near-surface air up. The O2 and N2 don't absorb the IR radiation from the hot surface, but they do physically contact it and warm up. Then go bouncing around off other molecules.
Heat release from condensation along with it. Basic heat transfer stuff I know you know well. Along with the obvious PV-work effects.
Posted on 3/20/19 at 1:17 pm to NC_Tigah
quote:
We know for example, H2O in the atmosphere is a very import radiative forcing agent or GHA. In fact it's postulated that water vapor accounts for up to 75% of the greenhouse effect.
Yes. Water vapor is an excellent greenhouse gas. The concentration of it in the atmosphere will also increase with warmer temperatures.
quote:
. . . And of course that is why deserts are so hot.
It does explain the large diurnal temperature range typical of deserts.
Posted on 3/20/19 at 1:20 pm to Kentucker
quote:
Signatories are approved for inclusion in the Petition Project list if they have obtained formal educational degrees at the level of Bachelor of Science or higher in appropriate scientific fields. The petition has been circulated only in the United States.
The current list of petition signers includes 9,029 PhD; 7,157 MS; 2,586 MD and DVM; and 12,715 BS or equivalent academic degrees. Most of the MD and DVM signers also have underlying degrees in basic science.
All of the listed signers have formal educations in fields of specialization that suitably qualify them to evaluate the research data related to the petition statement. Many of the signers currently work in climatological, meteorological, atmospheric, environmental, geophysical, astronomical, and biological fields directly involved in the climate change controversy.
The Petition Project classifies petition signers on the basis of their formal academic training, as summarized below. Scientists often pursue specialized fields of endeavor that are different from their formal education, but their underlying training can be applied to any scientific field in which they become interested.
Outlined below are the numbers of Petition Project signatories, subdivided by educational specialties. These have been combined, as indicated, into seven categories.
1. Atmospheric, environmental, and Earth sciences includes 3,805 scientists trained in specialties directly related to the physical environment of the Earth and the past and current phenomena that affect that environment.
2. Computer and mathematical sciences includes 935 scientists trained in computer and mathematical methods. Since the human-caused global warming hypothesis rests entirely upon mathematical computer projections and not upon experimental observations, these sciences are especially important in evaluating this hypothesis.
3. Physics and aerospace sciences include 5,812 scientists trained in the fundamental physical and molecular properties of gases, liquids, and solids, which are essential to understanding the physical properties of the atmosphere and Earth.
4. Chemistry includes 4,822 scientists trained in the molecular interactions and behaviors of the substances of which the atmosphere and Earth are composed.
5. Biology and agriculture includes 2,965 scientists trained in the functional and environmental requirements of living things on the Earth.
6. Medicine includes 3,046 scientists trained in the functional and environmental requirements of human beings on the Earth.
7. Engineering and general science includes 10,102 scientists trained primarily in the many engineering specialties required to maintain modern civilization and the prosperity required for all human actions, including environmental programs.
The following outline gives a more detailed analysis of the signers' educations.
Atmosphere, Earth, & Environment (3,805)
1. Atmosphere (579)
I) Atmospheric Science (112)
II) Climatology (39)
III) Meteorology (343)
IV) Astronomy (59)
V) Astrophysics (26)
2. Earth (2,240)
I) Earth Science (94)
II) Geochemistry (63)
III) Geology (1,684)
IV) Geophysics (341)
V) Geoscience (36)
VI) Hydrology (22)
3. Environment (986)
I) Environmental Engineering (487)
II) Environmental Science (253)
III) Forestry (163)
IV) Oceanography (83)
Computers & Math (935)
1. Computer Science (242)
2. Math (693)
I) Mathematics (581)
II) Statistics (112)
Physics & Aerospace (5,812)
1. Physics (5,225)
I) Physics (2,365)
II) Nuclear Engineering (223)
III) Mechanical Engineering (2,637)
2. Aerospace Engineering (587)
Chemistry (4,822)
1. Chemistry (3,129)
2. Chemical Engineering (1,693)
Biochemistry, Biology, & Agriculture (2,965)
1. Biochemistry (744)
I) Biochemistry (676)
II) Biophysics (68)
2. Biology (1,438)
I) Biology (1,049)
II) Ecology (76)
III) Entomology (59)
IV) Zoology (149)
V) Animal Science (105)
3. Agriculture (783)
I) Agricultural Science (296)
II) Agricultural Engineering (114)
III) Plant Science (292)
IV) Food Science (81)
Medicine (3,046)
1. Medical Science (719)
2. Medicine (2,327)
General Engineering & General Science (10,102)
1. General Engineering (9,833)
I) Engineering (7,280)
II) Electrical Engineering (2,169)
III) Metallurgy (384)
2. General Science (269)
I have a Chemical Engineering degree and I understand the molecular behavior of the relevant gasses. But I don't know shite about climate science. No way I would put my name on a petition either supporting or refuting man-made influence on global warming.
Posted on 3/20/19 at 1:25 pm to Duke
quote:
It does explain the large diurnal temperature range typical of deserts.
And places on the same latitude, same elevations, that receive the same radiation from the sun, same lack of ocean affects, but have drastic high temperature and drastic low temperature average differences, like Phoenix, AZ compared to Huntsville, AL. There is really only one difference, water vapor concentrations.
Posted on 3/20/19 at 1:40 pm to Duke
quote:I believe that's his point.
The O2 and N2 don't absorb the IR radiation from the hot surface, but they do physically contact it and warm up.
In fact, though the generally expressed contention is the atmosphere and GHGs warm the Earth, we know for a fact that is not exactly true, right?
By simply examining the moon, we know our atmosphere functions as both a heat shield and a terrestrial blanket. Otherwise we'd be exposed to enormous lunarlike temperature variance. Overheating for lack of IR protection, overcooling for lack of heat retention. To a certain degree we see that in deserts sans 75% of GHAs over wide expanses.
Likewise it is very difficult to fold the entire terrestrial temperature equation into the GHG box when nocturnal heat retention along with altitude effects are considered. Pressure variance almost certainly contributes.
Posted on 3/20/19 at 1:41 pm to Duke
quote:
The O2 and N2 don't absorb the IR radiation from the hot surface, but they do physically contact it and warm up.
So through conduction. I got that.
Hypothetical:
If we shine a very bright light on a control volume of pure N2 with the ability for that control volume to release energy to infinity will there be a temperature increase?
I hypothesize, yes. Yes there will be a temperature increase as a function of the energy output of the source, the number of N2 molecules per unit volume (density/pressure) and the heat capacity of the molecule.
Now if that control volume is a vacuum there will be no temperature to measure. If you are measuring temperature you are measuring the thermometer absorbing energy.
My point here is that you expect me to believe that N2 and O2 act like a vacuum when under radiative heat transfer (not conduction or convection). That is what I'm having difficulty with.
This post was edited on 3/20/19 at 2:02 pm
Posted on 3/20/19 at 1:46 pm to Dday63
quote:I'll bet you'd feel at least the same or perhaps more strongly if the claimed qualification was a background in Cognitive Science? Yet that is the background of one of the most outspoken proponents of the "AGW-consensus". It floats both ways.
I have a Chemical Engineering degree and I understand the molecular behavior of the relevant gasses. But I don't know shite about climate science. No way I would put my name on a petition either supporting or refuting man-made influence on global warming.
Posted on 3/20/19 at 2:07 pm to GumboPot
quote:Right. Diatomic gases play no role in radiative forcing, but that is not the only component of atmospheric heat production as you've noted. Pressure is certainly a component.
The O2 and N2 don't absorb the IR radiation from the hot surface, but they do physically contact it and warm up.
So though conduction. I got that.
Atmospheric pressure would not be expected to vary cyclically over time expanses though. For example, our ice age temp cycles would relate to something variable over the time course.
Posted on 3/20/19 at 2:19 pm to NC_Tigah
quote:
Diatomic gases play no role in radiative forcing, but that is not the only component of atmospheric heat production as you've noted. Pressure is certainly a component.
I know pressure is a small component, but a component nonetheless. That's generally why it's freezing cold in the mountains versus the beach. PV=nRT. Hold V, n, and R constant, when P increases so does T and when P decrease so does T. Relatively high P at the beach (or lower elevations) gets you higher Ts. Lower Ps on top of mountains gets you lower Ts.
Perfect example: Hawaii:

quote:
Atmospheric pressure would not be expected to vary cyclically over time expanses though.
Right.
quote:
For example, our ice age temp cycles would relate to something variable over the time course.
Ice ages are due to the earth orbital shape, and changes along it's axis, and wobble: Milankovitch Cycles. And solar activity.
This post was edited on 3/20/19 at 2:23 pm
Popular
Back to top



 1
1



