- My Forums
- Tiger Rant
- LSU Recruiting
- SEC Rant
- Saints Talk
- Pelicans Talk
- More Sports Board
- Fantasy Sports
- Golf Board
- Soccer Board
- O-T Lounge
- Tech Board
- Home/Garden Board
- Outdoor Board
- Health/Fitness Board
- Movie/TV Board
- Book Board
- Music Board
- Political Talk
- Money Talk
- Fark Board
- Gaming Board
- Travel Board
- Food/Drink Board
- Ticket Exchange
- TD Help Board
Customize My Forums- View All Forums
- Show Left Links
- Topic Sort Options
- Trending Topics
- Recent Topics
- Active Topics
Started By
Message
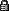
Titanium oxidizes at different colors depending on what voltage was run through it
Posted on 8/23/18 at 9:02 pm
Posted on 8/23/18 at 9:02 pm

Posted on 8/23/18 at 9:04 pm to DavidTheGnome
k... keep me oxidized.
Posted on 8/23/18 at 9:14 pm to DavidTheGnome
They have a few welders that make some sweet "art" by adjusting the heat when they weld the titanium. It's quite cool
Posted on 8/23/18 at 9:16 pm to DavidTheGnome
Actually really cool.
Posted on 8/23/18 at 9:16 pm to DavidTheGnome
Yep we’ve done it at work a good bit. Different voltages when the titanium is dipped in distilled water and TSP.
Posted on 8/23/18 at 9:20 pm to Spelt it rong
I googled there’s some cool looking stuff






Posted on 8/23/18 at 9:21 pm to shutterspeed
quote:
k... keep me oxidized
Posted on 8/23/18 at 9:24 pm to DavidTheGnome
Man, that is very cool! Strikes me as kind of odd that the colors are almost random as voltage varies linearly, though.
Posted on 8/23/18 at 9:28 pm to DavidTheGnome

You know I’m somewhat of a redditor myself.
Posted on 8/23/18 at 9:30 pm to starsandstripes
Actually, that is pretty cool
Posted on 8/23/18 at 9:30 pm to DavidTheGnome
As an commercial electrician for 20 yrs I have been “oxidized" in all those colors more than once.
Posted on 8/23/18 at 9:38 pm to DavidTheGnome
Titanium is one of my favorite things and I have almost nothing made of it.
Posted on 8/23/18 at 9:55 pm to DavidTheGnome
quote:
Titanium is one of the so-called reactive metals. This means that it reacts to certain conditions—current or heat, in this case—by developing an oxide layer that appears brightly colored, even though there is no pigment whatsoever. The color is, in a sense, an illusion. The layer varies in thickness according to the degree of heat or the amount of voltage. This oxide layer is quite chemically inert and quite permanent. It is also very thin and transparent. Its transparency allows light to bounce off of both the front and back of the oxide layer. Because the layer corresponds in thickness to wavelengths of light, the bounced light reinforces certain wavelengths (colors) and interferes with others. This causes the oxide layer to appear colored.
Back to top

 11
11













